-
-
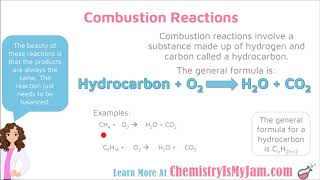
Combustion Reactions
-
-
-

-
Indicators of Chemical Change
-
-
-
-
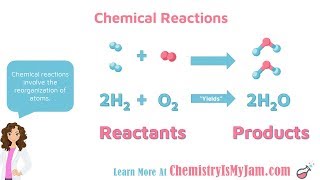
Chemical Reactions and Balancing Equations
-
-
-
-
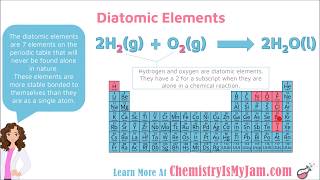
Diatomic Elements
-
-
-
-
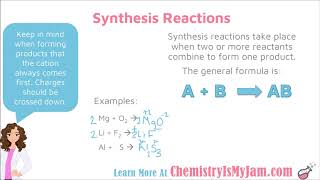
Synthesis and Decomposition Reactions
-
-
-
-
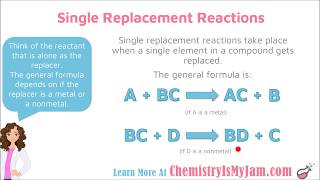
Single Replacement Reactions
-
-
-
-
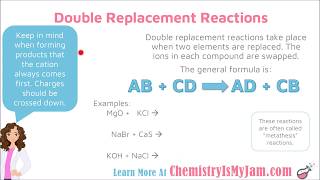
Double Replacement Reactions
-
-
-

-
Solubility Rules, Precipitation Reactions, and Net Ionic Equations
-
-
-
-
The Mole and Avogadro's Number
-
-
-

-
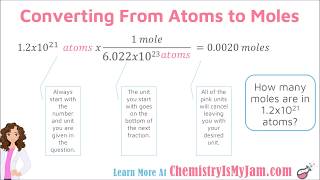
Mole Conversions
-
-
-
-
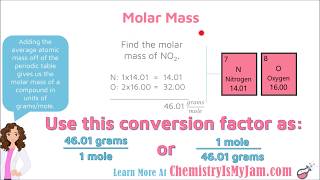
Molar Mass Video
-
-
-

-
Empirical and Molecular Formulas
-
-
-
-
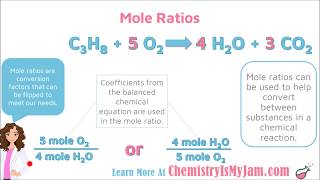
Mole Ratios and Stoichiometry
-
-
-
-
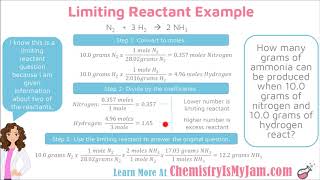
Limiting and Excess Reactants
-
-
-

-
Percent Yield
-
-
Need a Handout? Use one of the links below:
Chemical Change Indicators
Chemical Reactions and Balancing
Diatomic Elements
Synthesis and Decomposition Reactions
Single Replacement Reactions
Double Replacement Reactions
Solubility Rules, Precipitation Reactions, and Net Ionic Equations
Printable Solubility Rules
Combustion Reactions
The Mole and Using Avogadro’s Number
Mole Conversions
Molar Mass
Empirical and Molecular Formulas
Mole Ratios and Stoichiometry
Limiting Reactants
Percent Yield
