Welcome to Unit 1: Science and Measurement! Use the gallery below to read about important topics like the metric system, significant figures, valid measurements, and classifying matter. Each image contains a description of the concept along with a link to a video about the topic.
Prefer to jump straight into the videos? Click here for a video gallery about these topics.
If you love the images below and would like to customize them for your classroom, they are available for purchase as an editable PowerPoint. You can find them at my site’s shop or my TeachersPayTeachers page. Prices are the same in both places so that you can shop wherever you feel most comfortable.

Unit 1: Science and Measurement

What Is Science?

What Is Chemistry?

Steps In the Scientific Method

Scientific Theories

Scientific Laws

Quantitative and Qualitative Observations

Independent and Dependent Variables

Controlled Variables

Identify Variables
-
1

Unit 1: Science and Measurement
Unit one is titled “Science and Measurement.” This unit introduces the concept of chemistry, discusses the scientific method, reviews the metric system, discusses measurement and classifies matter. -
1

What Is Science?
What is science? Science is our effort to understand the world around us. We depend on observations, experiments, and measurements to make predictions about our world. Our understanding of the world is constantly changing as technology improves and new ideas are formed. -
0

What Is Chemistry?
Chemistry studies how and why atoms interact with one another. A chemist may look at what atoms make up a particular substance, like in forensics for example. They may determine what molecules need to be combined in order to produce a certain pharmaceutical product. They may predict the effect an industrial complex will have on the environment. These are just a few examples of the many contributions chemists can make. Chemistry is an experimental science. A chemist can’t look through a microscope and watch a chemical reaction take place. They follow the scientific method and make a series of observations in order to make any conclusions. -
1

Steps In the Scientific Method
The steps of the scientific method are identify a problem, background research, form a hypothesis, test the hypothesis, analyze data, and form a conclusion. This process is repeated until a hypothesis proves to be consistently correct. -
0

Scientific Theories
When using the scientific method, once a correct hypothesis proves itself through repeated testing, a scientific theory forms. Theories are much more common than scientific laws. -
0

Scientific Laws
Scientific laws are always true under the given conditions. There are often mathematical statements associated with scientific laws. Examples of scientific laws are the law of gravity, the law of conservation of mass, the laws of thermodynamics, the ideal gas law. -
0

Quantitative and Qualitative Observations
There are two major types of observations. Quantitative observations are measurable. They have a number and a unit associated with them. Qualitative observations are often more subjective. They do not have a number and a unit associated with them. Which do we prefer in science? Quantitative whenever possible. -
0

Independent and Dependent Variables
Independent variables are intentionally changed by the scientist. Dependent variables respond to changes in the independent variable. For example, changing the pH of a solution may be the independent variable, while measuring the color the solution turns with an indicator is the dependent variable. -
0

Controlled Variables
Controlled variables remain the same throughout the experiment. They help to make sure your experiment is measuring what you intend for it to measure. Ideally, in a controlled experiment, all variables will be controlled except the independent and dependent variables. -
2

Identify Variables
Identify the dependent, independent, and controlled variables in the following example. You are trying to determine which of three substances has the higher heat of solution. You add the same amount of each substance to the same amount of water at the same initial temperature. You measure the final temperature. The independent variable is changed intentionally by the scientist. In this case, the independent variable is the substance being tested. The dependent variable is what the scientists measures. In this case, the dependent variable is the final temperature. Controlled variables stay the same. The amount of substance, amount of water, and initial temperatures are all controlled variables. -
0

SI Units
Science uses the International System of Units, often called SI units. These units are used worldwide. The SI unit for length is the meter. The SI unit for mass is the gram. The SI unit for volume is the liter. The SI unit for time is the second. The SI unit for temperature is Kelvin. -
0

Temperature Scales
The Celsius and Kelvin temperature scales are frequently used in science. The Celsius scale is based on the freezing and boiling points of water. Water freezes at 0 degrees Celsius and boils at 100 degrees Celsius. The lowest point on the Celsius temperature scale is -273 degrees Celsius. This is called absolute zero and is the point when all molecular motion stops. In chemistry, we do calculations involving temperature and the large negative region on the Celsius temperature scale can cause problems with those calculations. The Kelvin scale fixes this problem. The Kelvin scale is shifted so that absolute zero is at 0 Kelvin. This puts the freezing point of water at 273 Kelvin and the boiling point of water at 373 kelvin. Fun fact: No degree sign is used on the kelvin scale. Some Jeopardy contestant is going to thank me for that one day. -
0

The Metric System
The metric system is based off of factors of 10. The original unit with no prefix is called the base. Base examples include the gram, liter, and meter. The prefixes for the meteric system include kilo (10^3), hector (10^2), deka (10^1), deci (10^-1), centi (10^-2), and milli (10^-3). -
1

Metric Conversions
An example of a metric conversion: Convert 5.28 millimiters to kilometers. Always start with the number and unit you are given in the question. The unit you start with goes on the bottom of the next fraction. Continue the pattern of putting the unit on top of the one fraction on the bottom of the next fraction. This will cause all units to cancel leaving you with the desired unit on the far right. -
1

Scientific Notation
Scientific notation is great for writing very large or very small numbers. A common number used in chemistry is 602 with 21 zeros after it. An easier way to write this is 6.02x10^23. When writing numbers in scientific notation, always put one number to the left of the decimal. The exponent tells how many places to move the decimal. Move the decimal to the right if the exponent is positive and to the left if the exponent is negative. -
0

Scientific Notation Examples
When writing numbers in scientific notation, always put one number to the left of the decimal. The exponent tells how many places to move the decimal. Move the decimal to the right if the exponent is positive and to the left if the exponent is negative. Only significant zeros are shown in scientific notation. For example, 0.000254 is 2.54x10^-4. The number 5420 is 5.42x10^3 in scientific notation. The number 0.0480 is 4.80x10^-2 in scientific notation. -
1

Accuracy Versus Precision
Measurements can be defined in terms of accuracy or precision. Accurate means close to the true value. Precise means several measurements are close together. A dart board where all of the darts have hit the bullseye is both accurate and precise. A dart board where all of the darts are close together but not in the bullseye is precise but not accurate. A dart board where darts are not on the bullseye and not close together is neither accurate nor precise. -
1

Accuracy Versus Precision Example
Measurements can be defined in terms of accuracy or precision. Accurate means close to the true value. Precise means several measurements are close together. Consider a sample that is known to have a mass of 5.0 grams. The sample is placed on the a scale three times. The scale reads 4.6 grams, 5.2 grams, and 4.2 grams. This scale is neither accurate nor precise. The measurements are not accurate because they are not close to their true value. They are not precise because they are not close together. The sample is placed on another scale three times. The scale reads 4.2 grams all three times. This scale is precise but not accurate. It is precise because the measurements are close together. It is not accurate because the measurements are not close to their true value. -
0

Valid Measurements
Laboratory equipment typically has lines that indicate certain measurements. In the graduated cylinder pictured, there is a line for every milliliter. A valid measurement reads all of the certain measurements possible and estimates one digit past the certain measurement. In this example, the volume of the liquid stops somewhere between 34 and 35 milliliters. A valid measurement would estimate one decimal point. For example, one person might read this volume as 34.8 milliliters while another person might read 34.9 milliliters. The 3 and the 4 are certain digits, while the one place after the decimal is the uncertain digit. All of these numbers are considered significant. It is expected in science that you estimate one digit. -
0

Purpose of Significant Figures
Measurements are only valid to a specific number of digits. Significant figures help scientists keep track of how valid the measurements are. For example, the ruler above only has four lines between each number. A measurement with this ruler could not be recorded as 3.25498 inches. There would be way to many significant figures. Recall that a valid measurement only has one estimated digit. -
1

Rules of Significant Figures
The rules for significant figures are: All non-zero digits are significant. All numbers between non-zero digits are significant. Leading zeros are never significant. Trailing zeros are significant ONLY if there is a decimal somewhere in the number. Leading zeros are the zeros before all non-zeros. Trailing zeros are the zeros after all non-zeros. -
0
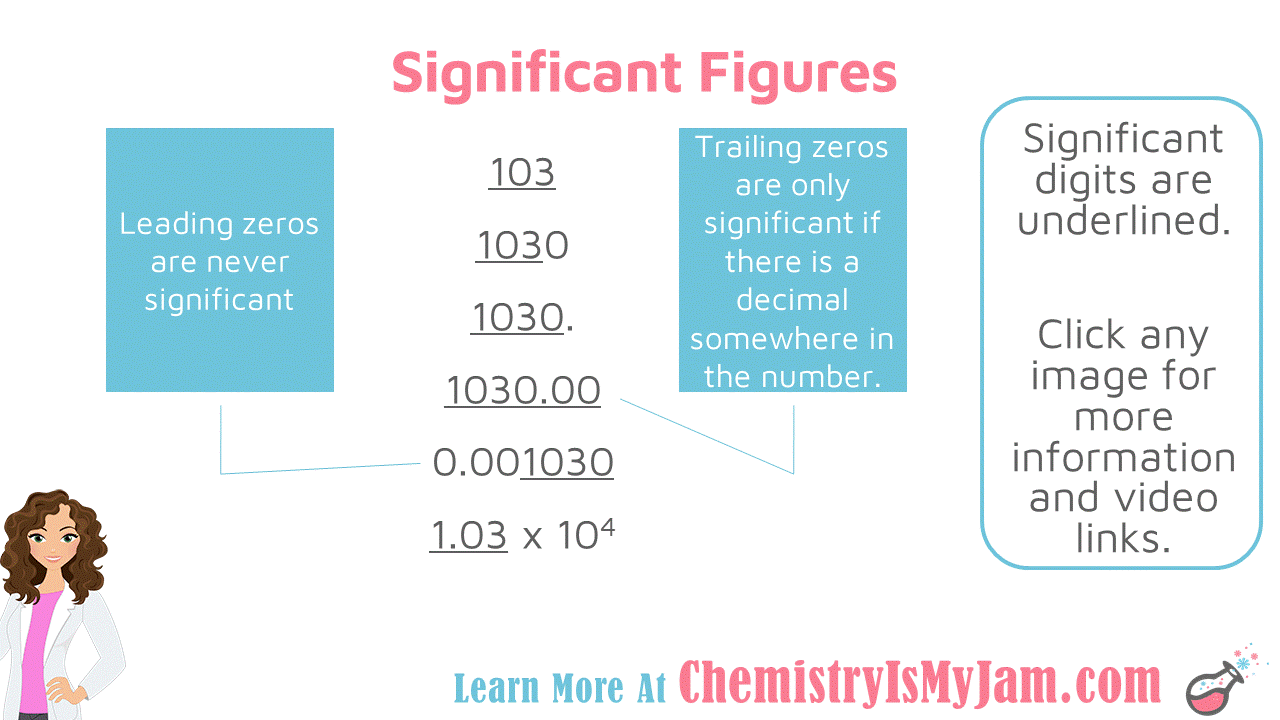
Significant Figure Examples
Some examples with significant figures. In the number 103, all of the numbers are significant. The 1 and the 3 are significant because they are non-zero. The zero in between is significant because all zeros between non-zeros are significant. There will never be a gap in your significant figures. They will always be next to one another. Let’s focus on leading and trailing zeros. Recall that leading zeros are before non-zeros and trailing zeros are after non-zeros. In the number 1030, the first three numbers are significant. The final zero is a trailing zero. It is not significant because there is not a decimal somewhere in the number. If there were a decimal at the end of the number, all four numbers would be significant. In the number 1030.00, all of the numbers are significant. The zeros at the end are trailing zeros. They are significant because there is a decimal somewhere in the number. In the number 0.001030, only the last four numbers are significant. The zeros at the beginning are leading zeros. Leading zeros are never significant. The trailing zero at the end is significant only because there is a decimal somewhere in the number. The number 1.03x10^4 is written in scientific notation. All of the digits before the multiplication sign will be significant in scientific notation. Non-significant zeros will not be included. This is another one of the advantages of scientific notation. Only significant digits are included. -
1

Adding and Subtracting Significant Figures
There are two sets of rules when doing math with significant figure. This image covers adding and subtracting. The next image will cover multiplying and dividing. For adding and subtracting, numbers should be rounded to the least place of significant figures. This means you should count the significant places after the decimal when adding and subtracting. For example, when 309.05 (which has two places after the decimal) is added to 432 (with no places after the decimal), the answer should be rounded to 822. Zero places after the decimal is smaller than two places after the decimal, so the answer should have zero places after the decimal. Another example: Consider 43.5 (one place after the decimal) minus 29.49 (two places after the decimal). The answer should be reported as 14.0 with one place after the decimal. Another example: Consider 1.054 (three places after the decimal) plus 29.49 (two places after the decimal). The answer should be reported as 4.03 with two places after the decimal. -
1

Multiplying and Dividing Significant Figures
There are two sets of rules when doing math with significant figure. This image covers multiplying and dividing. The previous image will covered adding and subtracting. For multiplying and dividing, numbers should be rounded to the least number of significant figures. This means you should count the number of significant figures in each of the numbers and round your answer to whichever is smaller. For example, when 39.2 (which has three significant figures) is multiplied by 403.45 (with five significant figures), the answer should be rounded to 1.58x10^4. Three significant figures is smaller than five significant figures, so the answer should have three significant figures. This example also demonstrates how sometimes it is helpful to write your answer in scientific notation in order to have the correct number of significant figures. Another example: Consider 2.90 (three significant figures) times 1.8 (two significant figures). The answer should be reported as 5.2 with two significant figures. Another example: Consider 490 (two significant figures) divided by 6 (one significant figure). The answer should be reported as 80 or 8x10^1. Both of these methods of reporting the answer only have one significant figure. -
0

Rounding Significant Figures
Rounding to the correct number of significant figures sometimes requires using scientific notation. Each of the following numbers will be rounded to four significant figures. For each, begin with the significant figure farthest to the left. Count four places to the right. Those are the digits that will be kept in the rounded number. 490.0006 rounds to 490.0. 0.029457 rounds to 0.02946. 182482 rounds to 1.825x10^5. -
0

Matter Definition
Matter is anything that has mass and takes up space. Examples of matter can be solid, liquid, or gas. Light and sound are not examples of matter. -
0

Classifying Matter
Matter is often classified as either a pure substance or a mixture. Mixtures are substances that are physically combined. They can be separated using physical means. A chemical reaction is not necessary to separate the parts of a mixture. Mixtures can be heterogeneous, meaning they are not the same throughout, or homogeneous, meaning they are the same throughout. Pure substances can be classified as either elements or compounds. An element consists of only one type of atom. The periodic table shows all of the elements that have been discovered so far. Compounds are two or more elements that have been chemically combined. A chemical reaction is necessary to separate the parts of a compound. -
0

Atoms
The smallest particles of an element are called atoms. Atoms of each type of element are unique. This means that carbon, gold, and hydrogen are all made up of different types of atoms. -
0

Compounds
Compounds are two or more elements chemically combined. A chemical reaction is necessary to separate the parts of a compound. Compounds have different properties than the elements that form them. For example, sodium chloride (or table salt) is made up of sodium and chlorine. Sodium is a metal that reacts dangerously with water. Chlorine is a poisonous gas. Together, they form a crystallized substance that is tasty on fries. -
0

Molecules
Molecules are groups of atoms that stay together as a unit. Molecules have a fixed ratio of atoms. Carbon dioxide, for example, always has two oxygen atoms and one carbon atom. -
0

Chemical Formulas
A chemical formula tells the kind and number of atoms in a molecule. In H2O, the H and O indicate that the elements hydrogen and oxygen are present. The 2 is a subscript. Subscripts are used to tell the number of each type of atom. They refer to the element directly to their left. If no subscripts are present, you can assume there is only one type of that element. A subscript outside of a set of parenthesis applies to all elements inside of the parenthesis. For example, in Mg(NO3)2, there is one magnesium atom, two nitrogen atoms, and six oxygen atoms. -
0

Capital Letters In Chemical Formulas
For chemical formulas, capital letters matter! The first letter in an element is capitalized. The second letter is not. Two capital letters in a row means two eparate elements. Co (with a capital C and lowercase O) is the element cobalt. It is a silver metal that is sometimes used in magnets. CO (with both letters capitalized) is the compound carbon monoxide. It is an odorless, poisonous gas. The only difference between these two examples is the capital letter. -
1

Homogeneous Versus Heterogeneous Mixtures
Mixtures can be classified as homogenous or heterogeneous. Homogenous mixtures are the same throughout. A sample of any two parts of the mixture would have the same composition. Homogenous mixtures are often called solutions. Heterogeneous mixtures are not evenly mixed. Different samples of the mixture would have different compositions. Mixtures are combined physically. Each substance keeps its original properties. The components of a mixture can be separated without a chemical reaction. Filtration (separation using difference in size) and distillation (separation using difference in boiling point) are often used to separate mixtures. -
1

Phases of Mixtures
Mixtures can be made up of combinations of solid, liquid, or gas. Air is a great example of a mixture of gases. Soft drinks demonstrate how a mixture can be a liquid and a gas. -
0

Chemical Properties
A chemical property describes the way a substance reacts with another substance. The act of measuring a chemical property causes a chemical change. Examples of chemical properties are reactivity, flammability, ability to spoil, rustability. -
0

Physical Properties
Physical properties describe the substance and can often be easily observed using human senses. A physical property can be measured without changing the composition of the substance. Examples of physical properties are color, texture, melting point, boiling point, viscosity, solubility, conductivity, and density. Note that melting and boiling point are physical properties. Changing the state of matter does not change the composition. Liquid water and ice are both made up of H2O. -
0
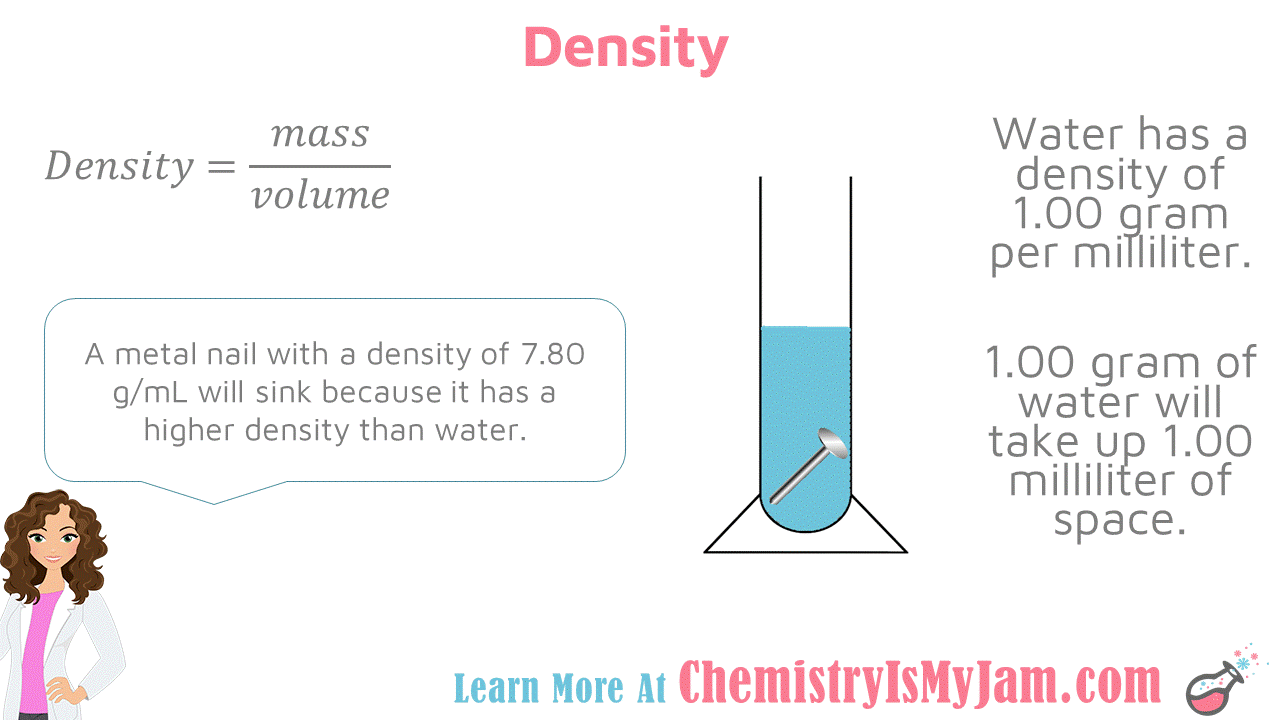
Density of Water
Density is mass divided by volume. Water has a density of 1.00 gram per milliliter. This means that 1.00 gram of water will take up 1.00 milliliter of space. A metal nail with a density of 7.80 grams per milliliter will sink because it has a higher density that water. An object with a lower density than water will float. -
1

Density Formula
The formula for density is Density equals mass divided by volume. This formula can be rearranged to solve for mass or volume. The density triangle helps to visualize these rearrangements. Covering up the variable you are solving for to see the necessary calculation. To solve for mass, multiply the density by the volume. To solve for volume, divide the mass by the density. -
0

Density Example
An example of a density problem: A piece of lead with a density of 11.34 grams per milliliter has a mass of 21.21 grams. What is the volume. Density is mass divided by volume. Plugging into that formula gives 11.34 equals 21.21 divided by x. You can cross multiply to solve, giving an answer of 1.870 milliliters. Note that sometimes volume is expressed in cubic centimeters (or cm^3). This is the same as 1 milliliter. -
0

Chemical Versus Physical Changes
There are two basic types of changes that occur in matter. In a physical change there is not change in the composition of the substance. Examples are changes in size, shape, or state of matter. For chemical changes, a new substance with a different composition is formed. Examples are spoiling, tarnishing, and rusting. -
0

Indicators of Chemical Change
How can you tell if a chemical reaction has taken place. Look for these clues: Color change, formation of a gas, formation of a solid (called a precipitate), or it may give off heat or light. -
0
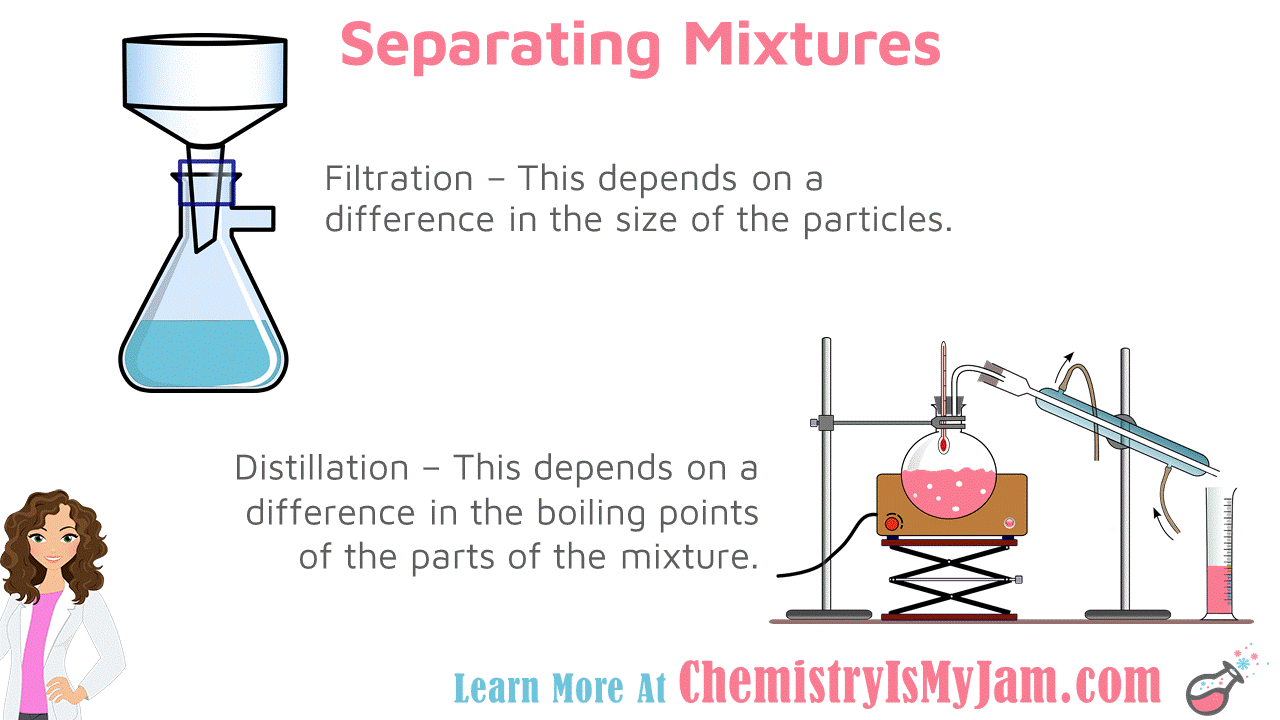
Separating Mixtures
Mixture can be separated using physical properties. Filtration depends on a difference in the size of the particles. Distillation depends on the difference in the boiling points of the parts of the mixture. In both cases, matter can be separated without a chemical reaction.
