Welcome to Unit 2: The Atom. Use the gallery below to learn about the parts of the atom, isotopes ions, the history of atomic theory, the Bohr model of the atom, energy levels of atoms, the electromagnetic spectrum, electron locations in terms of electron configuration, and orbital notation.
Each image contains a description of the concept along with a link to a video about the topic.
Prefer to jump straight into the videos? Click here for a video gallery about these topics.
If you love the images below and would like to customize them for your classroom, they are available for purchase as an editable PowerPoint. You can find them at my site’s shop or my TeachersPayTeachers page. Prices are the same in both places so that you can shop wherever you feel most comfortable.

Energy Sublevels - SPDF

Orbitals

Electrons Within Orbitals

Electron Configuration

Electron Configuration

Electron Configuration

Noble Gas Electron Configuration

Rules of Orbital Notation

Orbital Notation
-
1

Unit 2 - The Atom
Unit two is titled “The Atom.” This unit introduces the parts of the atom, explains isotopes and ions, describes the history of atomic theory, uses the Bohr model of the atom to understand energy levels of atoms, introduces the electromagnetic spectrum, describes electron locations in terms of electron configuration, and uses orbital notation to illustrate electrons in energy levels. -
1

Parts of the Atom
The atom has three subatomic particles. These are the tiny parts that make up the atom. They consist of positive protons inside the nucleus, neutral neutrons inside the nucleus, and negative electrons outside of the nucleus. -
0

The Nucleus
The nucleus is the dense portion in the center of the atom. Protons and neutrons are located inside the nucleus. Electrons are located outside of the nucleus. -
0

Parts of the Atom
The mass of subatomic particles is measured in amu, which stands for atomic mass unit. One amu is defined as 1/12 of the mass of carbon-12. Protons are located inside the nucleus. They are positive with a mass of 1 amu. Neutrons are located inside the nucleus. They are neutral with a mass of 1 amu. Electrons are located outside of the nucleus. They are negative and have a mass of 1/1800 amu. -
0

Differentiating Between Atoms
Atoms of each element are unique. The number of protons determines the element. The number of protons is called the atomic number. This is usually indicated at the top of each element block on the periodic table. The element ruthenium is number 44 on the periodic table. It has an atomic number of 44, meaning that it has 44 protons. The number indicated at the bottom of each element block on the periodic table is the average atomic mass. There is a difference between the mass number and the average atomic mass. Both of these will be discussed during this unit. -
0

Protons Differentiate Atoms
The number of protons in an atom determines the element. Carbon has an atomic number of 6, meaning it has 6 protons. Carbon will always have 6 protons. If an atom has something other than 6 protons, it is not carbon. Nitrogen has an atomic number of 7. It has 7 protons. Nitrogen must always have 7 protons in order to be nitrogen. -
0

Differentiating Between Atoms
How many protons are in an atom of gold? Gold has the symbol Au on the periodic table. It is element number 79, meaning the atomic number is 79. It has 79 protons. How many protons are in an atom of neon? Neon has the symbol Ne on the periodic table. It is element number 10, meaning the atomic number is 10. It has 10 protons. How many protons are in an atom of sodium? Sodium has the symbol Na on the periodic table. It is element number 11, meaning the atomic number is 11. It has 11 protons. -
0

Parts of the Atom
The mass number (often indicated by the symbol A) is the number of protons plus neutrons. Electrons are so small that their mass is ignored. Protons and neutrons both have a mass of 1 amu, while electrons are 1800 times smaller. -
0

Mass Number
Mass number is defined as the number of protons plus neutrons in an atom. This number is NOT found on the periodic table. The number on the periodic table is the average atomic mass and will be discussed later. The atoms of an element must all have the same number of protons in order to be that element. They can, however have a different number of neutrons, meaning they would have different mass numbers. An example would be that not all of the atoms of the element ruthenium have the same mass number. They must all have 44 protons, but they can have a different number of neutrons. -
0

Mass Number Examples
If carbon has a mass number of 13, how many protons and neutrons does it have? Carbon is element 6 on the periodic table, meaning it has 6 protons. The mass number tells the number of protons plus neutrons. 6 protons + some number of neutrons = a mass number of 13. This atom of carbon would have 7 neutrons. If boron has a mass number of 12, how many neutrons does it have? Boron is element number 5 on the periodic table, meaning it has 5 protons. The mass number tells the number of protons plus neutrons. 5 protons + some number of neutrons = a mass number of 11. This atom of boron would have 6 neutrons. -
0

Isotopes
Atoms with the same number of protons but different numbers of neutrons are called isotopes. In this example, two atoms are shown. They both have seven protons, meaning they are both atoms of nitrogen. The atom on the let has 7 neutrons while the atom on the right has 8 neutrons. They represent two different isotopes of nitrogen. -
0

Isotope Symbols
The symbol for an isotope is commonly written two ways. Consider the example of carbon with an atomic number of 6 and a mass number of 14. This can be written as “carbon-14” or “C” with a 6 on the bottom and a 14 on the top. The first method leaves out the atomic number. The atomic number can be determined from the fact that carbon is element 6 and will always have 6 protons. -
0

Isotopes
If you know the symbol for an isotope, you can determine the mass number, the protons, and the neutrons. The first example is oxygen-16. Oxygen always has 8 protons, meaning it always has an atomic number of 8. The mass number for this isotope of oxygen is 16. Subtracting the protons from the mass number (16-8) indicates that there are 8 neutrons. The second example gives us the atomic number and the neutrons. From the atomic number, we can look at the periodic table and see that the element is zinc (Zn). The atomic number also tells us that there are 30 protons. We are told that there are 35 neutrons. Adding 30 and 35 tells us that the mass number is 65, giving us a symbol of zinc-35. The final example is sodium-22. The fact that it is sodium tells us that there are 11 protons and that the atomic number is 11. The 22 tells us the mass number. Subtracting the atomic number from the mass number (22-11) indicates that there are 11 neutrons. -
0
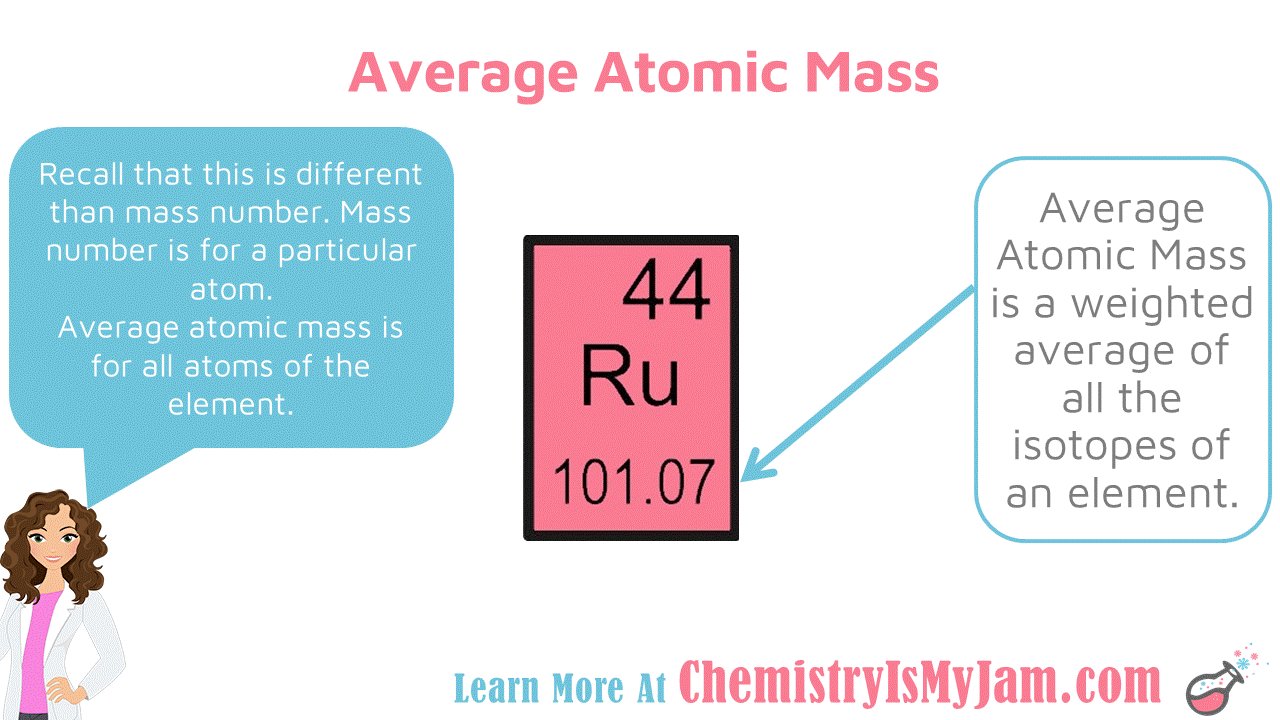
Average Atomic Mass
The number at the bottom of each block on the periodic table is the average atomic mass. This is a weighted average of all the isotopes of an element. For example, there are several isotopes of ruthenium. Each of them have a different mass number. The average atomic mass factors in all of the isotopes. The average atomic mass is different from the mass number. Mass number is for a particular atom. Average atomic mass is for all atoms of that element. -
0

Isotopes of Copper
Copper has two predominate isotopes. They are copper-63 and copper-65. There are more copper-63 isotopes than there are copper-65 isotopes, causing the average atomic mass of copper to be closer to 63 than it is to 65. It is a weighted average. The average atomic mass for copper is 63.546. -
0

Calculating Average Atomic Mass
Calculating average atomic mass is very similar to calculating your grade for a course. Your grade is a weighted average where one category generally counts more than others. In this example, tests count 50%, homework/classwork counts 30%, and labs count 20%. We can calculate your overall grade by converting those percentages into decimals and then multiplying each decimal by the grade for that type of assignment. For tests, we would multiply 0.50 by 92 to get 46. For homework/classwork, we would multiply 0.30 by 76 to get 23. For labs, we would multiple 0.20 by 95 to get 19. Adding those results together (46+23+19) gives a final course grade of 88. The grade was most influenced by the test average because that portion is weighted higher. -
0

Average Atomic Mass
Calculating average atomic mass is a weighted average. You need to know the actual mass of each isotope and the percent abundance of that isotope. Begin by converting percentages into decimals (fancy talk for moving the decimal two places to the left). The 69.15% becomes 0.6915. The 30.85% becomes 0.3085. These decimals then get multiplied by their respective masses. For copper-63, 0.6915 times 63.9296 gives 43.52. For copper-65, 0.3085 times 64.9278 gives 20.03. Adding these two together (43.52+20.03) gives the final average atomic mass of 63.55. Note that the average atomic mass of copper was closer to the value for copper-63 because this isotope had a higher percent abundance. That is what it means for this to be a weighted average. The isotope with the higher percent abundance influences the overall average atomic mass more. Note that some elements have more than two isotopes. You would carry out steps 1 and 2 for however many isotopes were present and then combine the answers for all isotopes. -
0

Electrons
Unless you are given a charge, you can assume that the number of protons and electrons in an atom match. This means you are assuming that the overall charge of the atom is zero and that there are equal numbers of positive and negative particles. Helium-4 has 2 protons because all atoms of helium must have 2 protons. We can assume that this atom of helium also has 2 electrons because no charge has been indicated. The number of neutrons can be determined by subtracting the mass number from the atomic number (4-2) to give us 2 neutrons. Soidum-25 has 11 protons because all atoms of sodium must have 11 protons. We can assume that this atom of sodium also has 11 electrons because no charge has been indicated. The number of neutrons can be determined by subtracting the mass number from the atomic number (25-11) to give us 14 neutrons. -
0

Cations and Anions
An atom with a charge is called an ion. Positive ions are called cations. Negative ions are called anions. Comparing the number of protons and electrons allows us to determine the charge of an ion. If there are more protons than electrons, the charge will be positive and the ion will be called a cation. If there are more electrons than protons, the charge will be negative and the ion will be called an anion. -
0

Ions
Comparing the number of protons and electrons in an atom allows us to determine the charge of the ion. For these examples, we will be determining the number of protons, neutrons, and electrons for several ions. The first example is nitrogen-14 with a charge of -3. We know that there are 7 protons by finding nitrogen on the periodic table and noting the atomic number. Subtracting the atomic number from the mass number (14-7) tells that the number of neutrons is 7. The charge of -3 indicates that there are three more negative particles (electrons) than there are positive particles (protons). If there are three more electrons than protons, and there are 7 protons, this ion must have 10 electrons. Another way to think of this is with an algebraic formula: electrons+charge=protons. The second example is lithium-7 with a charge of +1. We know that there are 3 protons by finding lithium on the periodic table and noting the atomic number. Subtracting the atomic number from the mass number (7-3) tells that the number of neutrons is 4. The charge of +1 indicates that there is one more positive particle (protons) than there are negative particles (electrons). If there is one more proton than electrons, and there are 3 protons, this ion must have 2 electrons. The third example is neon-20. We are not given the charge but we are told that there are 10 electrons. We know that there are 10 protons by finding neon on the periodic table and noting the atomic number. Subtracting the atomic number from the mass number (20-10) tells that the number of neutrons is 10. We can determine the charge by comparing the protons to the electrons. There are 10 protons and 10 electrons, meaning that the overall charge is 0 in this case. The final example tells us that there are 34 protons 45 neutrons, and 36 electrons. We can determine the element by finding element 34 on the periodic table. The element is selenium. We can determine the mass number by adding the protons and neutrons (34+45) to get a mass number of 79. We would write the isotope symbol as selenium-79. We can determine the charge by comparing the number of protons and electrons. There are 34 protons and 36 electrons. There are 2 more electrons than there are protons, meaning that there are 2 more negative particles than there are positive particles. This ion has a charge of -2. -
0

Democritus
Democritus was the first known person to discuss the concept of that atom. He was a Greek philosopher born in 460 BC. While the atom has not changed over time, our ideas about it have. His ideas about the atom said that matter is made up of atoms. Atoms are indestructible. Atoms are solid but invisible. Atoms are homogeneous. Atoms differ in size, shape, mass, position, and arrangement. He used the word Greek “atomos” for indivisible to describe the atom. -
0

John Dalton - Atomic Theory
John Dalton was born in England in 1776. He was a scientist famous for his atomic theory. Many of his beliefs about that atom align with what we currently know about the atom. He believed that all elements are made of atoms. Atoms of the same element are the same. Atoms of different elements are different. Atoms combine in small, whole number ratios. -
0

JJ Thomson - Cathode Ray Tube
Sir Joseph John Thomson, better known as JJ Thomson, was born in England in 1856. He discovered the electron using a cathode ray tube. He noticed a stream of charged particles that moved across the tube. The stream would move away from a negatively charged object and towards a positively charged object, leading to the conclusion that the particles were negative. JJ Thomson used this information to develop the Plum Pudding Model of the atom. -
0

JJ Thomson - Plum Pudding Model
JJ Thomson developed the Plum Pudding of the atom. He knew that the electron existed, but not where it was located. The nucleus had not been discovered at this time. The Plum Pudding Model of the atom said that the atom was mostly positive with regions of negative spread throughout. Plum pudding was a popular dish at the time made up of pudding (the positive) with chunks of plum (the negative). This could be compared to modern chocolate chip cookie dough where the dough is positive and the chips are negative. -
0

Ernest Rutherford - Gold Foil Experiment
Ernest Rutherford was born in 1871 in New Zealand. He used the gold foil experiment to discover the nucleus. The gold foil experiment projected positively charged particles, called alpha particles, at an extremely thin sheet of gold foil. A detector surrounded the foil so that the experiment would show where the alpha particles landed after interacting with the gold foil. At the time, scientists had not yet discovered the nucleus and the Plum Pudding Model of the atom was prevalent. With this in mind, Rutherford expected the particles to be deflected slightly as they passed through the foil. What actually happened is that most of the particles passed straight through the foil with little or no deflection. Some particles encountered something hard that threw them directly back the direction they came from. Rutherford was so surprised by this that he was quoted as saying: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” Rutherford realized that the atom was mostly empty space (allowing most of the alpha particles to go through undeflected) with a dense positive region in the center (causing the occasional particle to bounce straight back). Rutherford had discovered the nucleus. He knew that there was a dense positive region in the center of the atom with large amounts of empty space around it. Rutherford’s experiment caused scientists to totally change the model of the atom. It proved that the Plum Pudding model was not accurate. -
0

Neils Bohr - Planetary Model
Neils Bohr was born in Denmark in 1885. His model of the atom included a nucleus (thanks to the gold foil experiment) and energy levels where electrons were located. This model was called the Planetary Model of the atom. It assumed that electrons moved around the nucleus similar to how planets move around the sun. This model is very helpful for understanding that electrons fall into certain energy levels. It is not totally accurate in that electrons do not move in perfect circular orbits around the nucleus. -
0

Quantum Mechanical Model
The current model of the atom is attributed mostly to two German scientists: Werener Heisenberg and Erwin Schrodinger. This model is called the quantum mechanical model or the electron cloud model. This model of the atom uses probability to predict the location of the electron. Electrons are no longer believed to move in perfect circular orbits around the nucleus. There are regions of space, called orbitals, where the electrons are predicted to be located. This picture represents an electron density diagram. The circle in the center is the nucleus. The location of an electron was measured and plotted many times. Darker areas show where the electron is most likely to be located. In this case, the electron is in a spherical shaped orbital near the nucleus. Not all orbitals have this spherical shape. -
0

Quantized Electrons
The energy of the electron is quantized. This means that it can only increase or decrease by specific amounts. We call this amount a quantum of energy. You can think of this as stair steps. When a person climbs stairs, they can only climb by certain amounts. It is impossible to be standing halfway up a stair. Electrons reside in regions of space called orbitals. Electrons follow wave patterns within the orbitals. -
0

History of the Atom
This picture describes how our understanding of the atom has changed over time. Up through the early 1800’s, scientists knew that the atom existed and that it was a spherical shape, but they did not know about the particles within the atom. In the late 1800’s JJ Thomson discovered the electron and came up with the Plum Pudding Model of the Atom. In the early 1900’s, Ernest Rutherford carried out the gold foil experiment and discovered the nucleus. This lead to Bohr’s model of the atom. Bohr’s model also included energy levels for electrons. Over time, scientists realized that the electrons do not move in perfectly spherical orbits. Instead, they move in wave patterns and reside in regions of space called orbitals. This lead to the quantum mechanical model. -
0

Drawing Bohr Models
While scientists now recognize some limitations to the Bohr model, it still helps us make some valuable statements about the atoms. It is helpful for understanding the energy levels of an atom. Energy levels closer to the nucleus have lower energy. The number of electrons each energy levels holds follows the formula: 2n^2. This means that the first energy level holds 2 electrons, the second energy level holds 8 electrons, and the third energy level holds 18 electrons. -
0

Bohr Model Examples
For a neutral atom, the number of electrons will match the atomic number. Click the link to see a video of how to draw Bohr models for carbon, sulfur, nitrogen, and magnesium. Recall that the first energy level holds 2 electrons, the second energy level holds 8 electrons, and the third energy level holds 18 electrons. Carbon has 6 total electrons. Two will go in the first energy level and the remaining 4 will go in the second energy level. Sulfur has 16 total electrons. Two will go in the first energy level, 8 in the second energy level, and the remaining 6 will go in the third energy level. Nitrogen has 7 total electrons. Two will go in the first energy level and the remaining 5 will go in the second energy level. Magnesium has 12 total electrons. Two will go in the first energy level, 8 in the second energy level, and the remaining 2 will go in the third energy level. Click the video link to see these worked out. -
1

Parts of A Wave
Light moves as a wave. The highest point of a wave is called the crest and the lowest point is the trough. The distance from crest to crest, or from trough to trough is called the wavelength. Be careful not to measure from midpoint to midpoint when determining wavelength. This would result your measurement being half of what it should be. The amplitude is the height of the wave, starting from the midpoint and going to the crest. -
0

Electromagnetic Spectrum
The electromagnetic spectrum covers all types of light. The difference between the types of light is the wavelength. Speed remains the same for all types of light. Radio waves have the longest wavelength (10^3 meters). These are the size of buildings. Microwaves have a wavelength of 10^-2, roughly the size of a butterfly. Infrared waves have a wavelength of 10^-5, which is the size of a needle point. The visible region of the electromagnetic spectrum represents the portion that can be seen with the human eye. It covers all of the colors of the rainbow. The wavelength for the visible region is 0.5x10^-6 meters, which is around the size of a protozoan. Ultraviolet waves have a wavelength of 10^-8, which is the size of molecules. X rays have a wavelength of 10^-10, which is the size of atoms. Gamma waves are the smallest with a wavelength of 10^-12, which is the size of an atomic nucleus. On the electromagnetic spectrum pictured, wavelength decreases from left to right. Frequency represents the number of waves that pass a particular point in a second, measured in Hertz. Frequency and wavelength are inversely proportional. Frequence increases from left to right on the electromagnetic spectrum pictured here. This picture was provided by Inductiveload via Wikimedia Commons. -
0

Electromagnetic Spectrum Details
On the electromagnetic spectrum, wavelength normally decreases from left to right. Energy and frequency increase from left to right. Frequency is the number of waves that pass a particular point in a certain amount of time. Frequency and wavelength are inversely proportional. As one gets larger, the other gets smaller. The types of light on the electromagnetic spectrum differ by wavelength. Speed remains the same for all types of light. -
0

Bohr Models and Light
The lowest energy level possible for an electron is called the ground state. Excited electrons jump to higher energy levels. As soon as possible, they will fall back to their ground state and give off light. Electrons emit light when they fall from higher to lower energy levels. The Bohr model for hydrogen allows us to predict the type of light they give off. -
0

Bohr Model for Hydrogen
This is an illustration of the Bohr Model for hydrogen. It allows you to tell what type of light will be given off when electrons go from higher to lower energy levels. This particular table came from the North Carolina Chemistry Reference Tables. -
0

Bohr Model for Hydrogen Examples
What type of light is given off when an electron falls from the n=4 to the n=2 energy level? Find the arrow that starts on the n=4 energy level and ends on the n-2 energy level. This electron transition produces light with a wavelength of 486 nanometers, which is visible light. What type of light is given off when an electron falls from the n=3 to the n=1 energy level? This electron transition produces light with a wavelength of 103 nanometers, which is UV light. What type of light is given off when an electron falls from the n=5 to the n=3 energy level? This electron transition produces light with a wavelength of 1282 nanometers, which is IR light -
0

Locating Electrons
Locating electrons can be compared to locating a person in a stadium. You would find the person using a level number, a section number, and then a seat number. Each piece of information gets you closer to finding the correct person. Locating electrons involves knowing an energy level, an energy sublevel, an orbital, and then which of two possible electrons are within the orbital. -
1

Locating Electrons
Locating electrons begins with energy levels. Energy levels are indicated by the row on the periodic table. Within energy levels are sublevels. The four types of sublevels are S, P, D, and F. Within sublevels are orbitals. The type of sublevel dictates the number of orbitals. Within orbitals are electrons. Each orbital can hold up to two electrons. -
0

Energy Levels
Energy levels are indicated by the row on the periodic table. The Bohr model of aluminum shows that aluminum has three energy levels. This is confirmed by the fact that aluminum is on the third row down on the periodic table. -
0

Energy Sublevels - SPDF
There are four types of energy sublevels: S, P, D, and F. The S sublevel is spherical, has 1 orbital, and can hold up to 2 electrons. The P sublevel has a barbell shape, has 3 orbitals, and can hold up to 6 electrons. The D sublevel has 5 orbitals and can hold up to 10 electrons. The F sublevel has 7 orbitals and can hold up to 14 electrons. Note that the number of electrons is always twice the number of orbitals. This is because each orbital can hold up to 2 electrons. -
7

Orbitals
Within each sublevel are orbitals. These are regions of space where electrons are located. There are four energy sublevels within an atom: S, P, D, and F. Each energy sublevel has a different number of orbitals with different shapes. The S sublevel has 1 spherically shaped orbital. The P sublevel has 3 barbell shaped orbitals. Notice that when put on an three-dimensional axis, each orbital goes a different direction. The D sublevel has 5 orbitals. The F sublevel has 7 orbitals. The shapes of the D and F sublevels get more complicated than those of the S and P sublevels. -
0

Electrons Within Orbitals
Electrons are located in orbitals. Each orbital holds up to two electrons. They must have opposite spins. This is usually indicated by an up arrow and a down arrow to represent the two electrons. The need for opposite spins for the electrons relates to a physics concept. A spinning charge creates a magnetic field. Having a similar charge spinning the opposite direction would counteract the magnetic field. -
0

Electron Configuration
Electron configurations show where all of the electrons inside an atom are located. The shape of the periodic table helps us with writing electron configurations. The electron configuration for helium is 1s2. The 1 is the energy level. The s is the sublevel. The 2 tells us the number of electrons in that sublevel. -
1

Electron Configuration
When writing electron configurations, it makes more sense for helium to be next to hydrogen. The parts of the periodic table have been color coded to indicate which sublevel the outermost electrons in each element fall into. The first two columns make up the S bloc. The next 10 columns make up the D block and the 6 columns on the right make up the P block. The F block consists of the two rows beneath the main body of the periodic table. When writing an electron configuration, start at the top left and go through each element until you get to the element you are focused on. In the case of oxygen, the electron configuration is 1s22s22p4. -
0

Electron Configuration
Electron configuration is describing the location of each of the electrons in the atom in terms of energy levels and sublevels. Recall that the large number is the energy level, the letter is the sublevel, and the exponent is the number of electrons in that sublevel. The energy level is typically related to the period (or row down) on the periodic table. Notice that for the D block in the center of the table, the energy level decreases by 1. The F block technically fits into the bottom two rows on the periodic table. This becomes apparent when you follow the atomic numbers across the table. After 57, the atomic number jumps to 72 because 58-71 are in the F block. The F block is part of rows 6 and 7 on the periodic table, but the energy level drops by 2 in the F block. When writing an electron configuration, start at the top left and go through each element until you get to the element you are focused on. In the case of bromine, the electron configuration is 1s22s22p63s23p64s23d104p5. -
1

Noble Gas Electron Configuration
The short cut for writing electron configurations is to write the last noble gas you pass in brackets. Then begin writing electron configurations with the element immediately after that noble gas. Find bromine on the periodic table. From bromine, go up one row and all the way to the right. Argon is the last noble gas before bromine. The symbol for argon would go in brackets. The element following argon is sodium. Write electron configurations for sodium through bromine. The noble gas configuration for bromine is: [Ar]4s23d104p5 -
0

Rules of Orbital Notation
Orbital notation is another way to visualize the location of electrons on the periodic table. There are three rules that dictate the way electrons fill energy levels and sublevels. The Pauli Exclusion Principle says that electrons fill the lowest energy level first. In orbital notation, we will fill each sublevel before placing any electrons in the next sublevel. The Aufbau Principle says that each orbital can hold up to two electrons but they must have opposite spins. In orbital notation, arrows will be used to represent electrons. Each orbital can hold one up and one down arrow. Hund’s Rule says that electrons fill sublevels so that as many as possible will have the same spin. You can obey this rule with orbital notation by drawing all of the up arrows for a sublevel first, putting one up electron in each orbital before drawing any down electrons. -
0

Orbital Notation
Orbital notation uses either boxes or horizontal lines to represent orbitals and arrows to represent electrons. It is helpful to start with an electron configuration when first learning orbital notation. Recall that S sublevels have one orbital, P sublevels, have three orbitals, D sublevels have five orbitals, and F sublevels have seven orbitals. The electron configuration for oxygen is 1s22s22p4. The large number represents the energy level and the letter represents the sublevel. These energy levels and sublevels are written in order of lowest to highest energy. We will fill the lowest energy levels first, followed by the higher ones. Begin with the 1s sublevel. For an S sublevel, there should always be one orbital. In orbital notation, you should draw a box to represent the orbital and label it with the energy level and sublevel (1s). The 1s sublevel has two electrons, which can be determined by the exponent in electron configuration. These electrons must have opposite spins so the 1s box has one up and one down arrow. This is repeated for the 2s sublevel. For the 2p sublevel, three boxes should be drawn because P sublevels always have three orbitals. The P sublevel for oxygen has four electrons. Always draw all of the up arrows first. This means drawing an up arrow in each of the three 2p boxes before drawing the fourth arrow down.
