Welcome to Unit 3: The Periodic Table! Use the gallery below to read about important topics like the the history of the periodic table, the octet rule, as well as trends in ion charges, ionization energy, electronegativity, and atomic radius. Each image contains a description of the concept along with a link to a video about the topic.
Prefer to jump straight into the videos? Click here for a video gallery about these topics.
If you love the images below and would like to customize them for your classroom, they are available for purchase as an editable PowerPoint. You can find them at my site’s shop or my TeachersPayTeachers page. Prices are the same in both places so that you can shop wherever you feel most comfortable.

Electron Shielding

Electronegativity

Electronegativity Trend on the Periodic Table

How Atomic Radius is Measured

Atomic Radius Periodic Table Trend

Atomic Radius and Nuclear Charge
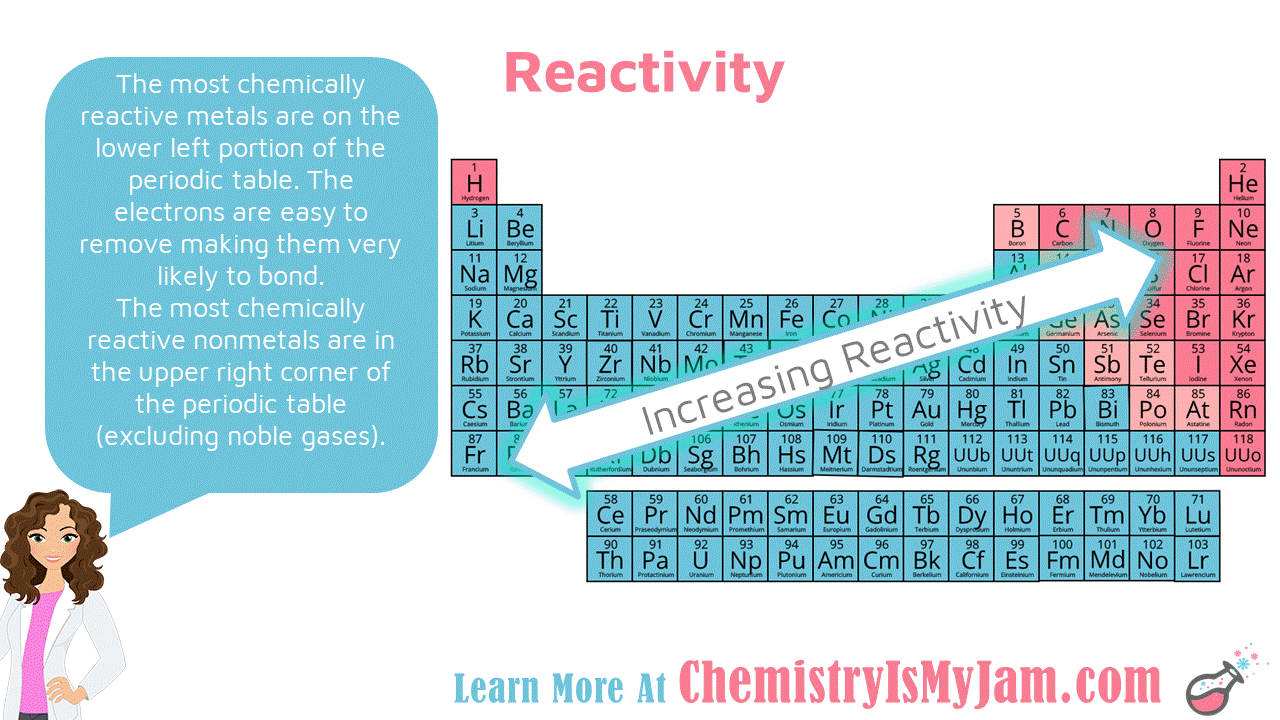
Reactivity of Metals and Nonmetals

Ion Size
-
3

History of the Periodic Table
The periodic table has only been developed over the last few centuries. Early civilizations were aware of common metals like silver and gold, but many centuries passed before more elements were discovered. In 1669, phosphorus was discovered by Hennig Brand. He discovered phosphorus while trying to turn human urine into gold. The 1700’s brought forth the discovery of many elemental gases. Common elements like sodium and calcium were discovered in the early 1800’s. Many radioactive elements were discovered in the late 1800’s. Efforts continue to discover larger elements. -
0

John Newland's Periodic Table Used the Law of Octaves
As more elements were discovered, a method of organizing them became necessary. Elements were arranged by weight. Properties seemed to repeat every 8 elements. John Newland, in 1863, noted that elements with similar properties often differed by a multiple of 8 in atomic weight. He proposed the law of octaves and divided the elements into 11 groups. -
0

Mendeleev and Meyer Worked on Separate Periodic Tables
Two chemists are sometimes referred to as the father of the periodic table: Dmitri Mendeleev from Russia and Lothar Meyer from Germany. These two men had very similar work but Mendeleev’s work was published first causing him to receive the credit for the periodic table. -
0

Mendeleev's Periodic Table
Mendeleev’s periodic table arranged the elements according to atomic weight. The groups that the elements fell into had similar properties. He left gaps in the table for elements that had not been discovered yet. He almost received the Nobel Prize for his work! The current periodic table is arranged by atomic number. You’ll find very few differences in the order of the elements. -
0

Mendeleev Left Blanks for Undiscovered Elements on His Periodic Table
Mendeleev left gaps in the table for elements that had not been discovered yet. He correctly predicted that gallium, scandium, and germanium would be discovered and left space in the table for them. -
1

Mendeleev Predicted the Properties of Gallium
Mendeleev accurately predicted the properties of Gallium years before it was discovered. This helped to validate his periodic table. -
0

Periodic Table Arranged by Atomic Number
The current periodic table is arranged by atomic number. This was a slight change from Mendeleev’s table, which was arranged by atomic mass. -
3

Groups and Periods on the Periodic Table
Horizontal rows are called periods. Elements in the same period have the same number of energy levels. Vertical columns are called groups. Elements in the same group have similar properties. -
2

Metals and Nonmetals on the Periodic Table
Metalloids separate the metals and the nonmetals. Metalloids have properties of both metals and nonmetals. The metalloids include boron, silicon, germanium, arsenic, antimony, tellurium, polonium, and astatine. Metals (shown here in blue) are located to the left of the metalloids, while nonmetals (shown here in pink) are located to the right. -
0

Properties of Metals and Nonmetals
Metals tend to be solid at room temperature, good conductors of heat and electricity, have luster (shiny), are malleable (bendable), and Are ductile (can be drawn into wires). Nonmetals tend to be gases at room temperature, poor conductors of heat and electricity, and brittle (break instead of bend). -
4
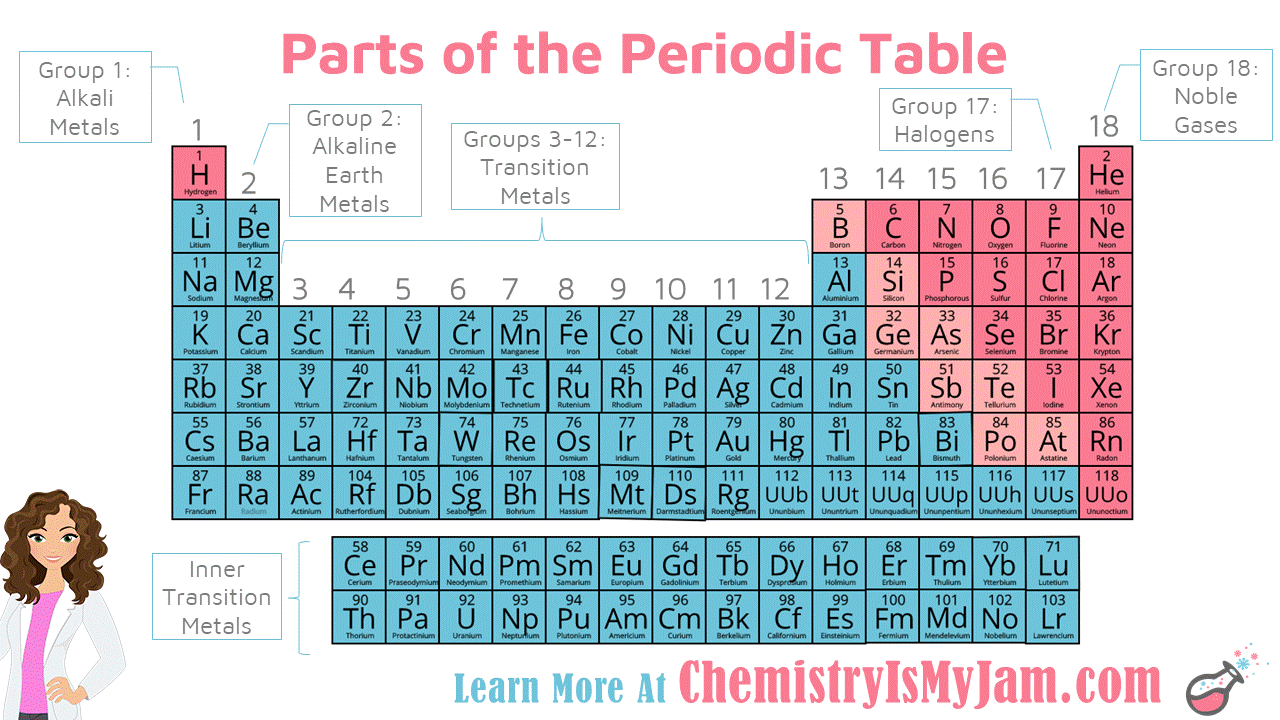
Notable Groups on the Periodic Table
There are certain groups on the periodic table that have names you should be aware of. Group 1 are the alkali metals, group 2 are the alkaline earth metals, groups 3-12 are the transition metals, group 17 are the halogens, group 18 are the noble gases, and the rows below the main body of the table are called the inner transition metals. -
0

The Octet Rule
The octet rule says that when elements form a chemical bond, they do so to achieve a full outer energy level. For most elements, this means achieving 8 valence electrons. This explains why noble gases are unreactive. They already have a full outer energy level. The bohr model of neon is shown. It has 8 electrons in the outermost energy level. -
1

How Metals and Nonmetals Achieve a Full Octet
Atoms can give away, take from, or share with other atoms in order to achieve a full octet. The electrons in the outermost energy level are called valence electrons. Metals have 4 or less valence electrons meaning that they tend to give away electrons in order to have a full octet in their outermost energy level. This causes the original outer energy level to disappear leaving the next full energy level down as the new outer energy level. Nonmetals have 4 or more electrons in their outermost energy level. They tend to take electrons from or share with other atoms in order to achieve a full octet in the outermost energy level. -
0

Valence Dot Diagrams
Valence dot diagrams are simple diagrams that only show the valence electrons for an atom. The valence dot electrons for the atoms in the third period on the periodic table are shown. Each one increases by one valence electron as you go from left to right across the table. This will help us understand an important trend for how atoms form ions. -
6

Ion Charges on the Periodic Table
Atoms achieve a full outer energy level by giving away or taking electrons. The charge of the ions they produce can be predicted. Take nitrogen for example. Nitrogen has 5 valence electrons. The easiest way for nitrogen to achieve a full outer energy level is to take 3 electrons from another atom, causing nitrogen to have a -3 charge. In the case of magnesium, which has 2 valence electrons, it is easier to give away the 2 electrons than to take 6 from another atom. This causes magnesium to have a +2 charge when it becomes an ion. As you go from left to right across the periodic table, the chares are +1, +2, +3, +/-4, -3, -2, -1, 0. This trend excludes the transition metals because these atoms can take multiple charges and do not follow the normal trend. -
0

Ions Resemble Nearest Noble Gas
When ions form, their electrons will resemble the electrons of the closest noble gas. For metals, that will be the noble gas before the ion. Sodium, for example, has 1 electron in its outer energy level (valence electron). It will give that electron away, causing the third energy level to disappear. This makes the electrons in the sodium ion resemble neon, the nearest noble gas. For nonmetals, that will be the noble gas after the ion. Fluorine, for example, has 7 valence electrons. The easiest way for it to achieve a full octet is to take one more electron from another atom. This causes the fluorine ion to resemble neon, the nearest noble gas. -
0

Electron Configuration of Ions
Electrons will fill the lowest energy level available. Oxygen gained 2 electrons when it became anion. They went into the lowest energy level available, which was the 2p sublevel. Electrons will be lost from the highest occupied energy level. Copper lost 1 electron when it become a cation. It came from the highest occupied energy level, which was the 4s sublevel. 4s is higher than 3d. Go by the number! -
0

Forces Between Charged Particles
The force (attraction or repulsion) between charged particles can be increased two ways: Increase the number of charged particles or decrease the distance between the particles. These attractions and repulsions will help explain some of the trends on the periodic table. -
0

Ionization Energy Trend on the Periodic Table
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. On the periodic table, ionization energy increases from left to right and decreases from top to bottom, meaning helium has the highest ionization energy. -
0

Ionization Energy
So much of chemistry can be explained in terms of attractions and repulsions positive and negative particles! Ionization energy increases from left to right because the nuclear charge (or number of protons) increases from left to right. This creates a stronger force pulling towards the nucleus. Ionization energy decreases from top to bottom because as you add energy levels, the electrons are getting farther from the nucleus. This lowers the force the electrons feel from the nucleus. -
0

Electron Shielding
Electrons are affected by forces from the nucleus as well as forces from other electrons. Electrons are affected by forces from the nucleus as well as forces from other electrons. An electron on the first energy level would be extremely difficult to remove. It is very close to the nucleus and is being pushed towards the nucleus by the outer electrons An electron on the outside energy level would be relatively easy to remove. It is farther from the nucleus. It is being repelled by the inner electrons. -
0

Electronegativity
Electronegativity is the ability of an atom in a molecule to attract shared electrons to itself. Simply speaking… When two atoms are bonded together, sometimes one of them has the ability to hog the electrons or pull them closer to itself. This atom is said to have a higher electronegativity. The trend in electronegativity does not include the noble gases. Noble gases do not bond because they already have their desired number of valence electrons. The element with the highest electronegativity value is fluorine. -
2

Electronegativity Trend on the Periodic Table
Electronegativity increases as you go from left to right on the periodic table. Electronegativity decreases as you go from top to bottom on the periodic table. Noble gases are usually ignored in this trend. -
0

How Atomic Radius is Measured
Atomic radius describes the size of the atom. The atom does not have an outer barrier making this difficult to measure. Atomic radius is defined as half of the distance between the nuclei in a molecule consisting of identical atoms. -
0

Atomic Radius Periodic Table Trend
Atomic radius decreases as you go from left to right on the periodic table. Atomic radius increases as you go from top to bottom on the periodic table. The largest element on the main body of the periodic table is francium. -
0

Atomic Radius and Nuclear Charge
So much of chemistry can be explained in terms of attractions and repulsions positive and negative particles! Atomic radius increases as you go down a group on the periodic table. This is because energy levels are being added. Atomic radius decreases as you go from left to right across the periodic table. This is because the increasing nuclear charge (or number of protons) pulls the electrons closer to the nucleus. -
2

Reactivity of Metals and Nonmetals
The most chemically reactive metals are on the lower left portion of the periodic table. The electrons are easy to remove making them very likely to bond. The most chemically reactive nonmetals are in the upper right corner of the periodic table (excluding noble gases). -
0

Ion Size
Parent atom size contributes to ion size. Cations are smaller than their parent atoms (they have given up an electron). Anions are larger than their parent atoms. Location on the periodic table also contributes to ion size: Ion size increases down a group. Ion size decreases from left to right with the exception of the sharp increase in size when switching from positive to negative charges.
